Trivial names of substances. Trivial names of some inorganic compounds What is the 2nd name
Classification of inorganic substances with examples of compounds
Now let's analyze the classification scheme presented above in more detail.
As we see, first of all, all inorganic substances are divided into simple And complex:
Simple substances These are substances that are formed by atoms of only one chemical element. For example, simple substances are hydrogen H2, oxygen O2, iron Fe, carbon C, etc.
Among simple substances there are metals, nonmetals And noble gases:
Metals formed by chemical elements located below the boron-astatine diagonal, as well as all elements located in side groups.
Noble gases formed by chemical elements of group VIIIA.
Nonmetals are formed respectively by chemical elements located above the boron-astatine diagonal, with the exception of all elements of side subgroups and noble gases located in group VIIIA:
The names of simple substances most often coincide with the names of the chemical elements whose atoms they are formed from. However, for many chemical elements the phenomenon of allotropy is widespread. Allotropy is the phenomenon when one chemical element is capable of forming several simple substances. For example, in the case of the chemical element oxygen, the existence of molecular compounds with the formulas O 2 and O 3 is possible. The first substance is usually called oxygen in the same way as the chemical element whose atoms it is formed, and the second substance (O 3) is usually called ozone. The simple substance carbon can mean any of its allotropic modifications, for example, diamond, graphite or fullerenes. The simple substance phosphorus can be understood as its allotropic modifications, such as white phosphorus, red phosphorus, black phosphorus.
Complex substances
Complex substances are substances formed by atoms of two or more chemical elements.
For example, complex substances are ammonia NH 3, sulfuric acid H 2 SO 4, slaked lime Ca (OH) 2 and countless others.
Among complex inorganic substances, there are 5 main classes, namely oxides, bases, amphoteric hydroxides, acids and salts:
Oxides - complex substances formed by two chemical elements, one of which is oxygen in the oxidation state -2.
The general formula of oxides can be written as E x O y, where E is the symbol of a chemical element.
Nomenclature of oxides
The name of the oxide of a chemical element is based on the principle:
For example:
Fe 2 O 3 - iron (III) oxide; CuO—copper(II) oxide; N 2 O 5 - nitric oxide (V)
You can often find information that the valence of an element is indicated in parentheses, but this is not the case. So, for example, the oxidation state of nitrogen N 2 O 5 is +5, and the valence, oddly enough, is four.
If a chemical element has a single positive oxidation state in compounds, then the oxidation state is not indicated. For example:
Na 2 O - sodium oxide; H 2 O - hydrogen oxide; ZnO - zinc oxide.
Oxides classification
Oxides, according to their ability to form salts when interacting with acids or bases, are divided accordingly into salt-forming And non-salt-forming.
There are few non-salt-forming oxides; they are all formed by nonmetals in the oxidation state +1 and +2. The list of non-salt-forming oxides should be remembered: CO, SiO, N 2 O, NO.
Salt-forming oxides, in turn, are divided into basic, acidic And amphoteric.
Basic oxides are those oxides that, when reacting with acids (or acidic oxides), form salts. Basic oxides include metal oxides in the oxidation state +1 and +2, with the exception of the oxides BeO, ZnO, SnO, PbO.
Acidic oxides These are oxides that, when reacting with bases (or basic oxides), form salts. Acidic oxides are almost all oxides of non-metals with the exception of non-salt-forming CO, NO, N 2 O, SiO, as well as all metal oxides in high oxidation states (+5, +6 and +7).
Amphoteric oxides are called oxides that can react with both acids and bases, and as a result of these reactions form salts. Such oxides exhibit a dual acid-base nature, that is, they can exhibit the properties of both acidic and basic oxides. Amphoteric oxides include metal oxides in the oxidation states +3, +4, as well as the oxides BeO, ZnO, SnO, and PbO as exceptions.
Some metals can form all three types of salt-forming oxides. For example, chromium forms the basic oxide CrO, the amphoteric oxide Cr 2 O 3 and the acidic oxide CrO 3.
As you can see, the acid-base properties of metal oxides directly depend on the degree of oxidation of the metal in the oxide: the higher the degree of oxidation, the more pronounced the acidic properties.
Grounds
Grounds - compounds with the formula Me(OH) x, where x most often equal to 1 or 2.
Exceptions: Be(OH) 2, Zn(OH) 2, Sn(OH) 2 and Pb(OH) 2 are not bases, despite the oxidation state of the metal +2. These compounds are amphoteric hydroxides, which will be discussed in more detail in this chapter.
Classification of bases
Bases are classified according to the number of hydroxyl groups in one structural unit.
Bases with one hydroxo group, i.e. type MeOH is called monoacid bases, with two hydroxo groups, i.e. type Me(OH) 2, respectively, diacid etc.
Bases are also divided into soluble (alkalis) and insoluble.
Alkalies include exclusively hydroxides of alkali and alkaline earth metals, as well as thallium hydroxide TlOH.
Nomenclature of bases
The name of the foundation is based on the following principle:
For example:
Fe(OH) 2 - iron (II) hydroxide,
Cu(OH) 2 - copper (II) hydroxide.
In cases where the metal in complex substances has a constant oxidation state, it is not required to indicate it. For example:
NaOH - sodium hydroxide,
Ca(OH) 2 - calcium hydroxide, etc.
Acids
Acids - complex substances whose molecules contain hydrogen atoms that can be replaced by a metal.
The general formula of acids can be written as H x A, where H are hydrogen atoms that can be replaced by a metal, and A is the acidic residue.
For example, acids include compounds such as H2SO4, HCl, HNO3, HNO2, etc.
Classification of acids
According to the number of hydrogen atoms that can be replaced by a metal, acids are divided into:
- O base acids: HF, HCl, HBr, HI, HNO 3 ;
- d basic acids: H 2 SO 4, H 2 SO 3, H 2 CO 3;
- T rehobasic acids: H 3 PO 4 , H 3 BO 3 .
It should be noted that the number of hydrogen atoms in the case of organic acids most often does not reflect their basicity. For example, acetic acid with the formula CH 3 COOH, despite the presence of 4 hydrogen atoms in the molecule, is not tetra- but monobasic. The basicity of organic acids is determined by the number of carboxyl groups (-COOH) in the molecule.
Also, based on the presence of oxygen in the molecules, acids are divided into oxygen-free (HF, HCl, HBr, etc.) and oxygen-containing (H 2 SO 4, HNO 3, H 3 PO 4, etc.). Oxygen-containing acids are also called oxoacids.
You can read more about the classification of acids.
Nomenclature of acids and acid residues
The following list of names and formulas of acids and acid residues is a must-learn.
In some cases, a number of the following rules can make memorization easier.
As can be seen from the table above, the construction of systematic names of oxygen-free acids is as follows:
For example:
HF—hydrofluoric acid;
HCl—hydrochloric acid;
H 2 S is hydrosulfide acid.
The names of acidic residues of oxygen-free acids are based on the principle:
For example, Cl - - chloride, Br - - bromide.
The names of oxygen-containing acids are obtained by adding various suffixes and endings to the name of the acid-forming element. For example, if the acid-forming element in an oxygen-containing acid has the highest oxidation state, then the name of such an acid is constructed as follows:
For example, sulfuric acid H 2 S +6 O 4, chromic acid H 2 Cr +6 O 4.
All oxygen-containing acids can also be classified as acid hydroxides because they contain hydroxyl groups (OH). For example, this can be seen from the following graphical formulas of some oxygen-containing acids:
Thus, sulfuric acid can otherwise be called sulfur (VI) hydroxide, nitric acid - nitrogen (V) hydroxide, phosphoric acid - phosphorus (V) hydroxide, etc. In this case, the number in brackets characterizes the degree of oxidation of the acid-forming element. This version of the names of oxygen-containing acids may seem extremely unusual to many, however, occasionally such names can be found in real KIMs of the Unified State Examination in Chemistry in tasks on the classification of inorganic substances.
Amphoteric hydroxides
Amphoteric hydroxides - metal hydroxides exhibiting a dual nature, i.e. capable of exhibiting both the properties of acids and the properties of bases.
Metal hydroxides in oxidation states +3 and +4 are amphoteric (as are oxides).
Also, as exceptions, amphoteric hydroxides include the compounds Be(OH) 2, Zn(OH) 2, Sn(OH) 2 and Pb(OH) 2, despite the oxidation state of the metal in them +2.
For amphoteric hydroxides of tri- and tetravalent metals, the existence of ortho- and meta-forms is possible, differing from each other by one water molecule. For example, aluminum(III) hydroxide can exist in the ortho form Al(OH)3 or the meta form AlO(OH) (metahydroxide).
Since, as already mentioned, amphoteric hydroxides exhibit both the properties of acids and the properties of bases, their formula and name can also be written differently: either as a base or as an acid. For example:
Salts
Salts - these are complex substances that contain metal cations and anions of acid residues.
For example, salts include compounds such as KCl, Ca(NO 3) 2, NaHCO 3, etc.
The definition presented above describes the composition of most salts, however, there are salts that do not fall under it. For example, instead of metal cations, the salt may contain ammonium cations or its organic derivatives. Those. salts include compounds such as, for example, (NH 4) 2 SO 4 (ammonium sulfate), + Cl - (methyl ammonium chloride), etc.
Also contradicting the definition of salts above is the class of so-called complex salts, which will be discussed at the end of this topic.
Classification of salts
On the other hand, salts can be considered as products of the replacement of hydrogen cations H + in an acid with other cations, or as products of the replacement of hydroxide ions in bases (or amphoteric hydroxides) with other anions.
With complete replacement, the so-called average or normal salt. For example, with complete replacement of hydrogen cations in sulfuric acid with sodium cations, an average (normal) salt Na 2 SO 4 is formed, and with complete replacement of hydroxide ions in the base Ca (OH) 2 with acidic residues of nitrate ions, an average (normal) salt is formed Ca(NO3)2.
Salts obtained by incomplete replacement of hydrogen cations in a dibasic (or more) acid with metal cations are called acidic. Thus, when hydrogen cations in sulfuric acid are incompletely replaced by sodium cations, the acid salt NaHSO 4 is formed.
Salts that are formed by incomplete replacement of hydroxide ions in two-acid (or more) bases are called bases. O strong salts. For example, with incomplete replacement of hydroxide ions in the base Ca(OH) 2 with nitrate ions, a base is formed O clear salt Ca(OH)NO3.
Salts consisting of cations of two different metals and anions of acidic residues of only one acid are called double salts. So, for example, double salts are KNaCO 3, KMgCl 3, etc.
If a salt is formed by one type of cations and two types of acid residues, such salts are called mixed. For example, mixed salts are the compounds Ca(OCl)Cl, CuBrCl, etc.
There are salts that do not fall under the definition of salts as products of the replacement of hydrogen cations in acids with metal cations or products of the replacement of hydroxide ions in bases with anions of acidic residues. These are complex salts. For example, complex salts are sodium tetrahydroxozincate and tetrahydroxoaluminate with the formulas Na 2 and Na, respectively. Complex salts can most often be recognized among others by the presence of square brackets in the formula. However, you need to understand that in order for a substance to be classified as a salt, it must contain some cations other than (or instead of) H +, and the anions must contain some anions other than (or instead of) OH -. So, for example, the compound H2 does not belong to the class of complex salts, since when it dissociates from cations, only hydrogen cations H + are present in the solution. Based on the type of dissociation, this substance should rather be classified as an oxygen-free complex acid. Likewise, the OH compound does not belong to salts, because this compound consists of cations + and hydroxide ions OH -, i.e. it should be considered a comprehensive foundation.
Nomenclature of salts
Nomenclature of medium and acid salts
The name of medium and acid salts is based on the principle:
If the oxidation state of a metal in complex substances is constant, then it is not indicated.
The names of acid residues were given above when considering the nomenclature of acids.
For example,
Na 2 SO 4 - sodium sulfate;
NaHSO 4 - sodium hydrogen sulfate;
CaCO 3 - calcium carbonate;
Ca(HCO 3) 2 - calcium bicarbonate, etc.
Nomenclature of basic salts
The names of the main salts are based on the principle:
For example:
(CuOH) 2 CO 3 - copper (II) hydroxycarbonate;
Fe(OH) 2 NO 3 - iron (III) dihydroxonitrate.
Nomenclature of complex salts
The nomenclature of complex compounds is much more complicated, and to pass the Unified State Exam you do not need to know much about the nomenclature of complex salts.
You should be able to name complex salts obtained by reacting alkali solutions with amphoteric hydroxides. For example:
*The same colors in the formula and name indicate the corresponding elements of the formula and name.
Trivial names of inorganic substances
By trivial names we mean the names of substances that are not related, or weakly related, to their composition and structure. Trivial names are determined, as a rule, either by historical reasons or by the physical or chemical properties of these compounds.
List of trivial names of inorganic substances that you need to know:
| Na 3 | cryolite |
| SiO2 | quartz, silica |
| FeS 2 | pyrite, iron pyrite |
| CaSO 4 ∙2H 2 O | gypsum |
| CaC2 | calcium carbide |
| Al 4 C 3 | aluminum carbide |
| KOH | caustic potassium |
| NaOH | caustic soda, caustic soda |
| H2O2 | hydrogen peroxide |
| CuSO 4 ∙5H 2 O | copper sulfate |
| NH4Cl | ammonia |
| CaCO3 | chalk, marble, limestone |
| N2O | laughing gas |
| NO 2 | brown gas |
| NaHCO3 | baking (drinking) soda |
| Fe3O4 | iron scale |
| NH 3 ∙H 2 O (NH 4 OH) | ammonia |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| SiC | carborundum (silicon carbide) |
| PH 3 | phosphine |
| NH 3 | ammonia |
| KClO3 | Bertholet's salt (potassium chlorate) |
| (CuOH)2CO3 | malachite |
| CaO | quicklime |
| Ca(OH)2 | slaked lime |
| transparent aqueous solution of Ca(OH) 2 | lime water |
| suspension of solid Ca(OH) 2 in its aqueous solution | lime milk |
| K2CO3 | potash |
| Na 2 CO 3 | soda ash |
| Na 2 CO 3 ∙10H 2 O | crystal soda |
| MgO | magnesia |
Chemical formula is an image using symbols.
Chemical element signs
Chemical sign or chemical element symbol– this is the first or two first letters of the Latin name of this element.
For example: FerrumFe , Cuprum –Cu , OxygeniumO etc.
Table 1: Information provided by a chemical sign
| Intelligence | Using the example of Cl |
| Item name | Chlorine |
| Non-metal, halogen | |
| One element | 1 chlorine atom |
| (Ar) of this element | Ar(Cl) = 35.5 |
| Absolute atomic mass of a chemical element
m = Ar 1.66 10 -24 g = Ar 1.66 10 -27 kg |
M (Cl) = 35.5 1.66 10 -24 = 58.9 10 -24 g |
The name of a chemical symbol in most cases is read as the name of a chemical element. For example, K – potassium, Ca – calcium, Mg – magnesium, Mn – manganese.
Cases when the name of a chemical symbol is read differently are given in Table 2:
| Chemical element name | Chemical sign | Chemical symbol name
(pronunciation) |
| Nitrogen | N | En |
| Hydrogen | H | Ash |
| Iron | Fe | Ferrum |
| Gold | Au | Aurum |
| Oxygen | O | ABOUT |
| Silicon | Si | Silicium |
| Copper | Cu | Cuprum |
| Tin | Sn | Stanum |
| Mercury | Hg | Hydrargium |
| Lead | Pb | Plumbum |
| Sulfur | S | Es |
| Silver | Ag | Argentum |
| Carbon | C | Tse |
| Phosphorus | P | Pe |
Chemical formulas of simple substances
The chemical formulas of most simple substances (all metals and many non-metals) are the signs of the corresponding chemical elements.
So iron substance And chemical element iron are designated the same - Fe .
If it has a molecular structure (exists in the form , then its formula is the chemical symbol of the element with index bottom right indicating number of atoms in a molecule: H 2, O2, O 3, N 2, F 2, Cl2, BR 2, P 4, S 8.
Table 3: Information provided by a chemical sign
| Intelligence | Using C as an example |
| Substance name | Carbon (diamond, graphite, graphene, carbyne) |
| Belonging of an element to a given class of chemical elements | Non-metal |
| One atom of an element | 1 carbon atom |
| Relative atomic mass (Ar) element that forms a substance | Ar(C) = 12 |
| Absolute atomic mass | M(C) = 12 1.66 10-24 = 19.93 10 -24 g |
| One substance | 1 mole of carbon, i.e. 6.02 10 23 carbon atoms |
| M (C) = Ar (C) = 12 g/mol |
Chemical formulas of complex substances
The formula of a complex substance is prepared by writing down the signs of the chemical elements of which the substance is composed, indicating the number of atoms of each element in the molecule. In this case, as a rule, chemical elements are written in order of increasing electronegativity in accordance with the following practical series:
Me, Si, B, Te, H, P, As, I, Se, C, S, Br, Cl, N, O, F
For example, H2O , CaSO4 , Al2O3 , CS 2 , OF 2 , NaH.
The exceptions are:
- some compounds of nitrogen with hydrogen (for example, ammonia NH 3 , hydrazine N 2H 4 );
- salts of organic acids (for example, sodium formate HCOONa , calcium acetate (CH 3COO) 2Ca) ;
- hydrocarbons ( CH 4 , C2H4 , C2H2 ).
Chemical formulas of substances existing in the form dimers (NO 2 , P2O 3 , P2O5, salts of monovalent mercury, for example: HgCl , HgNO3 etc.), written in the form N 2 O4,P 4 O6,P 4 O 10Hg 2 Cl2,Hg 2 ( NO 3) 2 .
The number of atoms of a chemical element in a molecule and a complex ion is determined based on the concept valency or oxidation states and is recorded index lower right from the sign of each element (index 1 is omitted). In this case, they proceed from the rule:
the algebraic sum of the oxidation states of all atoms in a molecule must be equal to zero (the molecules are electrically neutral), and in a complex ion - the charge of the ion.
For example:
2Al 3 + +3SO 4 2- =Al 2 (SO 4) 3
The same rule is used when determining the oxidation state of a chemical element using the formula of a substance or complex. It is usually an element that has several oxidation states. The oxidation states of the remaining elements forming the molecule or ion must be known.
The charge of a complex ion is the algebraic sum of the oxidation states of all the atoms that form the ion. Therefore, when determining the oxidation state of a chemical element in a complex ion, the ion itself is placed in brackets, and its charge is taken out of brackets.
When compiling formulas for valence a substance is represented as a compound consisting of two particles of different types, the valencies of which are known. Next they use rule:
in a molecule, the product of valence by the number of particles of one type must be equal to the product of valence by the number of particles of another type.
For example:
The number before the formula in a reaction equation is called coefficient. She indicates either number of molecules, or number of moles of substance.
The coefficient before the chemical symbol, indicates number of atoms of a given chemical element, and in the case when the sign is the formula of a simple substance, the coefficient indicates either number of atoms, or the number of moles of this substance.
For example:
- 3 Fe– three iron atoms, 3 moles of iron atoms,
- 2 H– two hydrogen atoms, 2 moles of hydrogen atoms,
- H 2– one molecule of hydrogen, 1 mole of hydrogen.
The chemical formulas of many substances have been determined experimentally, which is why they are called "empirical".
Table 4: Information provided by the chemical formula of a complex substance
| Intelligence | Using the example C aCO3 |
| Substance name | Calcium carbonate |
| Belonging of an element to a certain class of substances | Medium (normal) salt |
| One molecule of substance | 1 molecule calcium carbonate |
| One mole of substance | 6.02 10 23 molecules CaCO3 |
| Relative molecular mass of the substance (Mr) | Мr (CaCO3) = Ar (Ca) +Ar (C) +3Ar (O) =100 |
| Molar mass of the substance (M) | M (CaCO3) = 100 g/mol |
| Absolute molecular mass of the substance (m) | M (CaCO3) = Mr (CaCO3) 1.66 10 -24 g = 1.66 10 -22 g |
| Qualitative composition (what chemical elements form the substance) | calcium, carbon, oxygen |
| Quantitative composition of the substance: | |
| The number of atoms of each element in one molecule of a substance: | a calcium carbonate molecule is made up of 1 atom calcium, 1 atom carbon and 3 atoms oxygen. |
| The number of moles of each element in 1 mole of the substance: | In 1 mole CaCO 3(6.02 · 10 23 molecules) contained 1 mole(6.02 · 10 23 atoms) calcium, 1 mole(6.02 10 23 atoms) carbon and 3 mol(3 6.02 10 23 atoms) of the chemical element oxygen) |
| Mass composition of the substance: | |
| Mass of each element in 1 mole of substance: | 1 mole of calcium carbonate (100g) contains the following chemical elements: 40g calcium, 12g carbon, 48g oxygen. |
| Mass fractions of chemical elements in the substance (composition of the substance as a percentage by weight):
|
Composition of calcium carbonate by weight:
W (Ca) = (n (Ca) Ar (Ca))/Mr (CaCO3) = (1·40)/100= 0.4 (40%) W (C) = (n (Ca) Ar (Ca))/Mr (CaCO3) = (1 12)/100 = 0.12 (12%) W (O) = (n (Ca) Ar (Ca))/Mr (CaCO3) = (3 16)/100 = 0.48 (48%) |
| For a substance with an ionic structure (salt, acid, base), the formula of the substance provides information about the number of ions of each type in the molecule, their quantity and the mass of ions per 1 mole of the substance:
|
Molecule CaCO 3 consists of an ion Ca 2+ and ion CO 3 2-
1 mol ( 6.02 10 23 molecules) CaCO 3 contains 1 mol Ca 2+ ions And 1 mole of ions CO 3 2-; 1 mole (100g) of calcium carbonate contains 40g ions Ca 2+ And 60g ions CO 3 2- |
| Molar volume of a substance at standard conditions (for gases only) | |
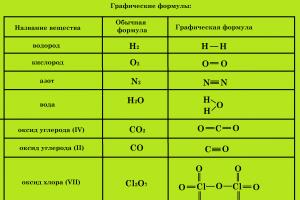
Graphic formulas
To obtain more complete information about a substance, use graphic formulas , which indicate order of connection of atoms in a molecule And valence of each element.
Graphic formulas of substances consisting of molecules sometimes, to one degree or another, reflect the structure (structure) of these molecules; in these cases they can be called structural .
To compile a graphical (structural) formula of a substance, you must:
- Determine the valence of all chemical elements that form the substance.
- Write down the signs of all chemical elements that form the substance, each in an amount equal to the number of atoms of a given element in the molecule.
- Connect the signs of chemical elements with dashes. Each dash denotes a pair that communicates between chemical elements and therefore belongs equally to both elements.
- The number of lines surrounding the sign of a chemical element must correspond to the valency of this chemical element.
- When formulating oxygen-containing acids and their salts, hydrogen atoms and metal atoms are bonded to the acid-forming element through an oxygen atom.
- Oxygen atoms are combined with each other only when formulating peroxides.
Examples of graphic formulas:
8.1. What is chemical nomenclature
Chemical nomenclature developed gradually over several centuries. As chemical knowledge accumulated, it changed several times. It is being refined and developed even now, which is connected not only with the imperfection of some nomenclature rules, but also with the fact that scientists are constantly discovering new and new compounds, which sometimes turn out to be named (and sometimes even put together formulas), using existing rules. impossible. The nomenclature rules currently accepted by the scientific community around the world are contained in a multi-volume publication: “IUPAC Nomenclature Rules for Chemistry”, the number of volumes in which is continuously increasing.
You are already familiar with the types of chemical formulas, as well as some of the rules for their composition. What are the names of chemical substances?
Using nomenclature rules, you can create systematic Name substances.
For many substances, in addition to systematic ones, traditional, so-called trivial titles. When they appeared, these names reflected certain properties of substances, methods of production, or contained the name of what the substance was isolated from. Compare the systematic and trivial names of the substances given in Table 25.
All names of minerals (natural substances that make up rocks) are also trivial, for example: quartz (SiO 2); rock salt, or halite (NaCl); zinc blende, or sphalerite (ZnS); magnetic iron ore, or magnetite (Fe 3 O 4); pyrolusite (MnO 2); fluorspar, or fluorite (CaF 2) and many others.
Table 25. Systematic and trivial names of some substances
Systematic name |
Trivial name |
|
| NaCl | Sodium chloride | Table salt |
| Na 2 CO 3 | Sodium carbonate | Soda, soda ash |
| NaHCO3 | Sodium bicarbonate | Baking soda |
| CaO | Calcium oxide | Quicklime |
| Ca(OH)2 | Calcium hydroxide | Slaked lime |
| NaOH | Sodium hydroxide | Caustic soda, caustic soda, caustic |
| KOH | Potassium hydroxide | Caustic potassium |
| K2CO3 | Potassium carbonate | Potash |
| CO2 | Carbon dioxide | Carbon dioxide, carbon dioxide |
| CO | Carbon monoxide | Carbon monoxide |
| NH4NO3 | Ammonium nitrate | Ammonium nitrate |
| KNO 3 | Potassium nitrate | Potassium nitrate |
| KClO3 | Potassium chlorate | Bertholet's salt |
| MgO | Magnesium oxide | Magnesia |
For some of the most well-known or widespread substances, only trivial names are used, for example: water, ammonia, methane, diamond, graphite and others. In this case, such trivial names are sometimes called special.
You will learn how the names of substances belonging to different classes are composed in the following paragraphs.
| Sodium carbonate Na 2 CO 3 . The technical (trivial) name is soda ash (that is, calcined) or simply “soda.” The white substance, thermally very stable (melts without decomposition), dissolves well in water, partially reacting with it, and an alkaline environment is created in the solution. Sodium carbonate is an ionic compound with a complex anion, the atoms of which are linked together by covalent bonds. Soda was previously widely used in everyday life for washing clothes, but has now been completely replaced by modern washing powders. Sodium carbonate is obtained using a rather complex technology from sodium chloride, and is used mainly in the production of glass. Potassium carbonate K 2 CO 3. The technical (trivial) name is potash. In structure, properties and use, potassium carbonate is very similar to sodium carbonate. Previously, it was obtained from plant ash, and the ash itself was used in washing. Currently, most potassium carbonate is obtained as a by-product of the production of alumina (Al 2 O 3), used to make aluminum. Due to its hygroscopicity, potash is used as a drying agent. It is also used in the production of glass, pigments, and liquid soap. In addition, potassium carbonate is a convenient reagent for obtaining other potassium compounds. |
CHEMICAL NOMENCLATURE, SYSTEMATIC NAME, TRIVIAL NAME, SPECIAL NAME.
1. Write down ten trivial names of any compounds (not in the table) from the previous chapters of the textbook, write down the formulas of these substances and give their systematic names.
2. What do the trivial names “table salt”, “soda ash”, “carbon monoxide”, “burnt magnesia” mean?
8.2. Names and formulas of simple substances
The names of most simple substances coincide with the names of the corresponding elements. Only all allotropic modifications of carbon have their own special names: diamond, graphite, carbyne and others. In addition, one of the allotropic modifications of oxygen has its own special name - ozone.
The simplest formula of a simple non-molecular substance consists only of the symbol of the corresponding element, for example: Na - sodium, Fe - iron, Si - silicon.
Allotropic modifications are designated using alphabetic indices or letters of the Greek alphabet:
C (a) – diamond; -
Sn – gray tin;
C (gr) – graphite; -
Sn – white tin.
In the molecular formulas of molecular simple substances, the index, as you know, shows the number of atoms in the molecule of the substance:
H 2 – hydrogen; O 2 – oxygen; Cl 2 – chlorine; O 3 – ozone.
In accordance with nomenclature rules, the systematic name of such a substance must contain a prefix indicating the number of atoms in the molecule:
H 2 – dihydrogen;
O 3 – trioxygen;
P 4 – tetraphosphorus;
S 8 - octasulfur, etc., but at present this rule has not yet become generally accepted.
Table 26.Numeric prefixes
| Factor | Prefix | Factor | Prefix | Factor | Prefix |
| mono | penta | nona | |||
| di | hexa | soundboard | |||
| three | hepta | Undeka | |||
| tetra | Octa | dodeca |
| Ozone O3– a light blue gas with a characteristic odor, in a liquid state it is dark blue, in a solid state it is dark purple. This is the second allotropic modification of oxygen. Ozone is much more soluble in water than oxygen. O 3 is unstable and even at room temperature slowly turns into oxygen. Very reactive, destroys organic substances, reacts with many metals, including gold and platinum. You can smell ozone during a thunderstorm, since in nature ozone is formed as a result of the action of lightning and ultraviolet radiation on atmospheric oxygen. Above the Earth there is an ozone layer located at an altitude of about 40 km, which traps the bulk of the ultraviolet radiation of the Sun, which is destructive for all living things. Ozone has bleaching and disinfecting properties. In some countries it is used to disinfect water. In medical institutions, ozone produced in special devices - ozonizers - is used to disinfect premises. |
8.3. Formulas and names of binary substances
In accordance with the general rule, in the formula of a binary substance, the symbol of an element with a lower electronegativity of atoms is placed in the first place, and in the second place - with a higher one, for example: NaF, BaCl 2, CO 2, OF 2 (and not FNa, Cl 2 Ba, O 2 C or F 2 O!).
Since electronegativity values for atoms of different elements are constantly being refined, two rules of thumb are usually used:
1. If a binary compound is a compound of a metal-forming element with
element forming a non-metal, then the symbol of the element forming the metal is always placed in first place (on the left).
2. If both elements included in the compound are elements that form non-metals, then their symbols are arranged in the following sequence:
B, Si, C, Sb, As, P, N, H, Te, Se, S, At, I, Br, Cl, O, F.
Note: It should be remembered that nitrogen's place in this practical series does not correspond to its electronegativity; as a general rule it should be placed between chlorine and oxygen.
Examples: Al 2 O 3, FeO, Na 3 P, PbCl 2, Cr 2 S 3, UO 2 (according to the first rule);
BF 3, CCl 4, As 2 S 3, NH 3, SO 3, I 2 O 5, OF 2 (according to the second rule).
The systematic name of a binary compound can be given in two ways. For example, CO 2 can be called carbon dioxide - you already know this name - and carbon monoxide (IV). In the second name, the Stock number (oxidation state) of carbon is indicated in parentheses. This is done in order to distinguish this compound from CO - carbon monoxide (II).
You can use either type of name, depending on which one is more convenient in this case.
Examples (more convenient names are highlighted):
| MnO | manganese monoxide | manganese(II) oxide |
| Mn2O3 | dimanganese trioxide | manganese oxide(III) |
| MnO2 | manganese dioxide | manganese(IV) oxide |
| Mn2O7 | dimanganese heptoxide | manganese oxide(VII) |
Other examples:
If the atoms of the element that comes first in the formula of a substance exhibit only one positive oxidation state, then neither numerical prefixes nor the designation of this oxidation state in the name of the substance are usually used, for example:
Na 2 O – sodium oxide; KCl – potassium chloride;
Cs 2 S – cesium sulfide; BaCl 2 – barium chloride;
BCl 3 – boron chloride; HCl – hydrogen chloride (hydrogen chloride);
Al 2 O 3 – aluminum oxide; H 2 S – hydrogen sulfide (hydrogen sulfide).
1. Make up systematic names of substances (for binary substances - in two ways):
a) O 2, FeBr 2, BF 3, CuO, HI;
b) N 2, FeCl 2, Al 2 S 3, CuI, H 2 Te;
c) I 2, PCl 5, MnBr 2, BeH 2, Cu 2 O.
2.Name each of the nitrogen oxides in two ways: N 2 O, NO, N 2 O 3, NO 2, N 2 O 4, N 2 O 5. Emphasize more user-friendly names.
3. Write down the formulas of the following substances:
a) sodium fluoride, barium sulfide, strontium hydride, lithium oxide;
b) carbon(IV) fluoride, copper(II) sulfide, phosphorus(III) oxide, phosphorus(V) oxide;
c) silicon dioxide, diiodine pentoxide, diphosphorus trioxide, carbon disulfide;
d) hydrogen selenide, hydrogen bromide, hydrogen iodide, hydrogen telluride;
e) methane, silane, ammonia, phosphine.
4. Formulate the rules for compiling formulas for binary substances according to the position of the elements that make up this substance in the system of elements.
8.4. Formulas and names of more complex substances
As you have already noticed, in the formula of a binary compound, the first place is the symbol of a cation or atom with a partial positive charge, and the second is the symbol of an anion or an atom with a partial negative charge. Formulas for more complex substances are compiled in the same way, but the places of atoms or simple ions in them are taken by groups of atoms or complex ions.
As an example, consider the compound (NH 4) 2 CO 3. In it, the formula of the complex cation (NH 4) is in first place, and the formula of the complex anion (CO 3 2) is in second place.
In the formula of the most complex ion, the symbol of the central atom, that is, the atom to which the remaining atoms (or groups of atoms) of this ion are associated, is placed first, and the oxidation state of the central atom is indicated in the name.
Examples of systematic names:
Na 2 SO 4 sodium tetraoxosulfate(VI),
K 2 SO 3 potassium(II) trioxosulfate(IV),
CaCO 3 calcium(II) trioxocarbonate(IV),
(NH 4) 3 PO 4 ammonium tetraoxophosphate(V),
PH 4 Cl phosphonium chloride,
Mg(OH) 2 magnesium(II) hydroxide.
Such names accurately reflect the composition of the compound, but are very cumbersome. Therefore, abbreviated ones ( semi-systematic) names of these compounds:
Na 2 SO 4 sodium sulfate,
K 2 SO 3 potassium sulfite,
CaCO 3 calcium carbonate,
(NH 4) 3 PO 4 ammonium phosphate,
Mg(OH) 2 magnesium hydroxide.
The systematic names of acids are composed as if the acid is a hydrogen salt:
H 2 SO 4 hydrogen tetraoxosulfate(VI),
H 2 CO 3 hydrogen trioxocarbonate (IV),
H 2 hydrogen hexafluorosilicate (IV). (You will learn about the reasons for using square brackets in the formula of this compound later)
But for the most well-known acids, nomenclature rules allow the use of their trivial names, which, together with the names of the corresponding anions, are given in Table 27.
Table 27.Names of some acids and their anions
Name |
Formula
SEMI-SYSTEMATIC NAMES OF ACIDS AND SALTS. | |||
Check information. It is necessary to check the accuracy of the facts and reliability of the information presented in this article. On the talk page there is a discussion on the topic: Doubts regarding terminology. Chemical formula ... Wikipedia
A chemical formula reflects information about the composition and structure of substances using chemical symbols, numbers and dividing symbols of brackets. Currently, the following types of chemical formulas are distinguished: The simplest formula. Can be obtained by experienced... ... Wikipedia
A chemical formula reflects information about the composition and structure of substances using chemical symbols, numbers and dividing symbols of brackets. Currently, the following types of chemical formulas are distinguished: The simplest formula. Can be obtained by experienced... ... Wikipedia
A chemical formula reflects information about the composition and structure of substances using chemical symbols, numbers and dividing symbols of brackets. Currently, the following types of chemical formulas are distinguished: The simplest formula. Can be obtained by experienced... ... Wikipedia
A chemical formula reflects information about the composition and structure of substances using chemical symbols, numbers and dividing symbols of brackets. Currently, the following types of chemical formulas are distinguished: The simplest formula. Can be obtained by experienced... ... Wikipedia
Main article: Inorganic compounds List of inorganic compounds by element informational list of inorganic compounds presented in alphabetical order (by formula) for each substance, hydrogen acids of the elements (if ... ... Wikipedia
This article or section needs revision. Please improve the article in accordance with the rules for writing articles... Wikipedia
A chemical equation (equation of a chemical reaction) is a conventional representation of a chemical reaction using chemical formulas, numerical coefficients and mathematical symbols. The equation of a chemical reaction gives qualitative and quantitative... ... Wikipedia
Chemical software are computer programs used in the field of chemistry. Contents 1 Chemical editors 2 Platforms 3 Literature ... Wikipedia
Books
- Japanese-English-Russian dictionary for installation of industrial equipment. About 8,000 terms, Popova I.S.. The dictionary is intended for a wide range of users and primarily for translators and technical specialists involved in the supply and implementation of industrial equipment from Japan or...
- A brief dictionary of biochemical terms, Kunizhev S.M.. The dictionary is intended for students of chemical and biological specialties at universities studying a course in general biochemistry, ecology and fundamentals of biotechnology, and can also be used in ...
The formula for the basis of life - water - is well known. Its molecule consists of two hydrogen atoms and one oxygen, which is written as H2O. If there is twice as much oxygen, then a completely different substance will be obtained - H2O2. What is it and how will the resulting substance differ from its “relative” water?
H2O2 - what is this substance?
Let's look at it in more detail. H2O2 is the formula of hydrogen peroxide, Yes, the same one that is used to treat scratches, white. Hydrogen peroxide H2O2 - scientific.
For disinfection, use a three percent peroxide solution. In pure or concentrated form, it causes chemical burns to the skin. A thirty percent peroxide solution is otherwise called perhydrol; Previously, it was used in hairdressers to bleach hair. The skin burned by it also turns white.
Chemical properties of H2O2
Hydrogen peroxide is a colorless liquid with a “metallic” taste. It is a good solvent and easily dissolves in water, ether, and alcohols.
Three and six percent peroxide solutions are usually prepared by diluting a thirty percent solution. When storing concentrated H2O2, the substance decomposes with the release of oxygen, so it should not be stored in tightly sealed containers to avoid an explosion. As the peroxide concentration decreases, its stability increases. Also, to slow down the decomposition of H2O2, you can add various substances to it, for example, phosphoric or salicylic acid. To store solutions of high concentration (more than 90 percent), sodium pyrophosphate is added to peroxide, which stabilizes the state of the substance, and aluminum vessels are also used.

H2O2 can be both an oxidizing agent and a reducing agent in chemical reactions. However, more often peroxide exhibits oxidizing properties. Peroxide is considered to be an acid, but a very weak one; salts of hydrogen peroxide are called peroxides.
as a method of producing oxygen
The decomposition reaction of H2O2 occurs when the substance is exposed to high temperature (more than 150 degrees Celsius). As a result, water and oxygen are formed.
Reaction formula - 2 H2O2 + t -> 2 H2O + O2
The oxidation state of H in H 2 O 2 and H 2 O = +1.
Oxidation state of O: in H 2 O 2 = -1, in H 2 O = -2, in O 2 = 0
2 O -1 - 2e -> O2 0
O -1 + e -> O -2
2 H2O2 = 2 H2O + O2
Hydrogen peroxide can also decompose at room temperature if a catalyst (a chemical that speeds up the reaction) is used.
In laboratories, one of the methods for producing oxygen, along with the decomposition of bertholite salt or potassium permanganate, is the decomposition reaction of peroxide. In this case, manganese (IV) oxide is used as a catalyst. Other substances that accelerate the decomposition of H2O2 are copper, platinum, and sodium hydroxide.
History of the discovery of peroxide
The first steps towards the discovery of peroxide were taken in 1790 by the German Alexander Humboldt, when he discovered the transformation of barium oxide into peroxide when heated. That process was accompanied by the absorption of oxygen from the air. Twelve years later, scientists Tenard and Gay-Lussac conducted an experiment on burning alkali metals with excess oxygen, resulting in sodium peroxide. But hydrogen peroxide was obtained later, only in 1818, when Louis Thénard studied the effect of acids on metals; a low amount of oxygen was necessary for their stable interaction. Conducting a confirmatory experiment with barium peroxide and sulfuric acid, the scientist added water, hydrogen chloride and ice to them. After a short time, Tenar discovered small frozen drops on the walls of the container with barium peroxide. It became clear that this was H2O2. Then they gave the resulting H2O2 the name “oxidized water.” This was hydrogen peroxide - a colorless, odorless, difficult-to-evaporate liquid that dissolves other substances well. The result of the interaction of H2O2 and H2O2 is a dissociation reaction, peroxide is soluble in water.

An interesting fact is that the properties of the new substance were quickly discovered, allowing it to be used in restoration work. Tenard himself, using peroxide, restored a painting by Raphael that had darkened with time.
Hydrogen peroxide in the 20th century
After a thorough study of the resulting substance, it began to be produced on an industrial scale. At the beginning of the twentieth century, electrochemical technology for the production of peroxide, based on the process of electrolysis, was introduced. But the shelf life of the substance obtained by this method was short, about a couple of weeks. Pure peroxide is unstable, and for the most part it was produced in a thirty percent concentration for bleaching fabrics and in a three or six percent concentration for household needs.
Scientists in Nazi Germany used peroxide to create a liquid-fuel rocket engine, which was used for defense purposes in World War II. As a result of the interaction of H2O2 and methanol/hydrazine, powerful fuel was obtained, on which the aircraft reached speeds of more than 950 km/h.
Where is H2O2 used now?
- in medicine - for treating wounds;
- in the pulp and paper industry the bleaching properties of the substance are used;
- in the textile industry, natural and synthetic fabrics, furs, and wool are bleached with peroxide;
- as rocket fuel or its oxidizer;
- in chemistry - to produce oxygen, as a foaming agent for the production of porous materials, as a catalyst or hydrogenating agent;
- for the production of disinfectants or cleaning agents, bleaches;
- for bleaching hair (this is an outdated method, since hair is severely damaged by peroxide);

Hydrogen peroxide can be successfully used to solve various household problems. But only three percent hydrogen peroxide can be used for these purposes. Here are some ways:
- To clean surfaces, you need to pour peroxide into a container with a spray bottle and spray it on contaminated areas.
- To disinfect objects, they need to be wiped with an undiluted H2O2 solution. This will help cleanse them of harmful microorganisms. Washing sponges can be soaked in water with peroxide (1:1 ratio).
- To bleach fabrics, add a glass of peroxide when washing white items. You can also rinse white fabrics in water mixed with a glass of H2O2. This method restores whiteness, protects fabrics from yellowing and helps remove stubborn stains.
- To combat mold and mildew, mix peroxide and water in a 1:2 ratio in a container with a spray bottle. Spray the resulting mixture onto contaminated surfaces and after 10 minutes clean them with a brush or sponge.
- You can renew darkened grout in tiles by spraying peroxide on the desired areas. After 30 minutes, you need to thoroughly rub them with a stiff brush.
- To wash dishes, add half a glass of H2O2 to a full basin of water (or a sink with a closed drain). Cups and plates washed in this solution will shine clean.
- To clean your toothbrush, you need to dip it in an undiluted three percent peroxide solution. Then rinse under strong running water. This method disinfects hygiene items well.
- To disinfect purchased vegetables and fruits, you should spray a solution of 1 part peroxide and 1 part water on them, then rinse them thoroughly with water (can be cold).
- At your dacha, you can use H2O2 to fight plant diseases. You need to spray them with a peroxide solution or soak the seeds shortly before planting in 4.5 liters of water mixed with 30 ml of forty percent hydrogen peroxide.
- To revive aquarium fish, if they are poisoned by ammonia, suffocated when the aeration is turned off, or for another reason, you can try placing them in water with hydrogen peroxide. You need to mix three percent peroxide with water at the rate of 30 ml per 100 liters and place lifeless fish in the resulting mixture for 15-20 minutes. If they do not come to life during this time, then the remedy did not help.

Even as a result of vigorously shaking a bottle of water, a certain amount of peroxide is formed in it, since the water is saturated with oxygen during this action.
Fresh fruits and vegetables also contain H2O2 until they are cooked. When heating, cooking, frying and other processes with accompanying high temperatures, a large amount of oxygen is destroyed. This is why cooked foods are considered not so healthy, although some vitamins remain in them. Freshly squeezed juices or oxygen cocktails served in sanatoriums are useful for the same reason - due to oxygen saturation, which gives the body new strength and cleanses it.
Danger of peroxide when ingested
After the above, it may seem that peroxide can be specifically taken orally, and this will benefit the body. But this is not true at all. In water or juices, the compound is found in minimal quantities and is closely associated with other substances. Taking “unnatural” hydrogen peroxide internally (and all peroxide purchased in a store or produced as a result of chemical experiments independently cannot be considered natural, and moreover, has too high a concentration compared to natural) can lead to dangers to life and health consequences. To understand why, we need to turn again to chemistry.
As already mentioned, under certain conditions, hydrogen peroxide breaks down and releases oxygen, which is an active oxidizing agent. can occur when H2O2 collides with peroxidase, an intracellular enzyme. The use of peroxide for disinfection is based on its oxidizing properties. So, when a wound is treated with H2O2, the released oxygen destroys living pathogenic microorganisms that have entered it. It has the same effect on other living cells. If you treat intact skin with peroxide and then wipe the treated area with alcohol, you will feel a burning sensation, which confirms the presence of microscopic damage after peroxide. But when low concentration peroxide is used externally, there will be no noticeable harm to the body.

It’s another matter if you try to take it orally. That substance, which can damage even relatively thick skin on the outside, ends up on the mucous membranes of the digestive tract. That is, chemical mini-burns occur. Of course, the released oxidizing agent - oxygen - can also kill harmful microbes. But the same process will happen with the cells of the food tract. If burns as a result of the action of the oxidizing agent are repeated, then atrophy of the mucous membranes is possible, and this is the first step on the path to cancer. The death of intestinal cells leads to the body's inability to absorb nutrients, which explains, for example, weight loss and the disappearance of constipation in some people who practice “treatment” with peroxide.
Separately, it is necessary to say about this method of using peroxide, such as intravenous injections. Even if for some reason they were prescribed by a doctor (this can only be justified in case of blood poisoning, when there are no other suitable drugs available), then under medical supervision and with strict dosage calculations, there are still risks. But in such an extreme situation, this will be a chance for recovery. Under no circumstances should you prescribe hydrogen peroxide injections to yourself. H2O2 poses a great danger to blood cells - red blood cells and platelets, since it destroys them when it enters the bloodstream. In addition, a fatal blockage of blood vessels by the released oxygen can occur - a gas embolism.
Safety precautions for handling H2O2
- Keep out of the reach of children and disabled persons. The lack of odor and distinct taste makes peroxide especially dangerous for them, since large doses can be taken. If the solution gets inside, the consequences of use can be unpredictable. You should consult a doctor immediately.
- Peroxide solutions with a concentration of more than three percent cause burns if they come into contact with the skin. The burn area should be washed with plenty of water.

- Do not allow the peroxide solution to get into your eyes, as this will cause swelling, redness, irritation, and sometimes pain. First aid before contacting a doctor is to wash the eyes generously with water.
- Store the substance in such a way that it is clear that it is H2O2, that is, in a container with a sticker to avoid accidental use for other purposes.
- Storage conditions that prolong its life are a dark, dry, cool place.
- Hydrogen peroxide should not be mixed with any liquids other than clean water, including chlorinated tap water.
- All of the above applies not only to H2O2, but also to all preparations containing it.